A groundbreaking advancement in rheumatoid arthritis (RA) treatment may soon transform the way millions of patients manage their condition. Scientists at the Institute of Nano Science and Technology (INST) in Mohali have developed a novel “self-actuating” drug delivery system that releases medication only when inflammation is present in the joints. This intelligent system could offer more effective, targeted relief while reducing side effects and the need for frequent drug administration.
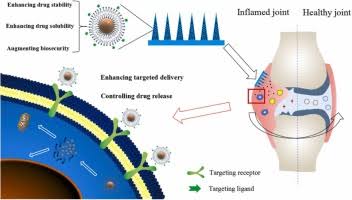 RA is a chronic autoimmune disorder that causes persistent joint inflammation, severe pain, and irreversible damage. Current treatments often involve systemic drug administration, which can lead to significant side effects and requires frequent dosing to maintain effectiveness. Many patients struggle with long-term management, as conventional medications are rapidly cleared from the body and fail to provide sustained relief. Addressing these challenges, researchers have designed a smart drug delivery system that directly responds to the biochemical signals in inflamed joints, ensuring that medication is released precisely when and where it is needed.
RA is a chronic autoimmune disorder that causes persistent joint inflammation, severe pain, and irreversible damage. Current treatments often involve systemic drug administration, which can lead to significant side effects and requires frequent dosing to maintain effectiveness. Many patients struggle with long-term management, as conventional medications are rapidly cleared from the body and fail to provide sustained relief. Addressing these challenges, researchers have designed a smart drug delivery system that directly responds to the biochemical signals in inflamed joints, ensuring that medication is released precisely when and where it is needed.
The system employs specially designed microspheres loaded with methotrexate, a widely used anti-rheumatic drug. These microspheres are engineered to detect inflammation by sensing elevated levels of specific enzymes—MMP-2 and MMP-9—that become more active during RA flare-ups. Once these enzymes reach a certain threshold, the drug is released in a controlled manner, reducing joint swelling and pain while promoting tissue repair. This approach minimizes unnecessary exposure to medication, lowering the risk of side effects and improving overall treatment outcomes.
The research team, led by Dr. Rahul Kumar Verma, has developed a formulation consisting of polymer-lipid hybrid micro-composites. The lipid component, derived from soya lecithin, ensures high drug encapsulation efficiency, while the polymer component, gelatin, responds to inflammatory enzymes. This innovative design allows for a pulsatile drug release mechanism, meaning the medication is only activated when needed. By responding to the body’s natural biochemical signals, this system offers a more precise and safer alternative to traditional RA treatments.
Published in the journal Biomaterial Advances, the study highlights the system’s remarkable success in preclinical trials. Animal studies demonstrated significant reductions in joint swelling, inflammation, and cartilage damage, along with improved joint repair. More importantly, the ability to sustain drug retention in affected joints means fewer doses are required, leading to improved patient compliance and a better quality of life.
Beyond RA, this technology has the potential to revolutionize treatments for other inflammatory diseases, such as synovitis and inflammatory bowel disease. It could also open new possibilities for smart biomaterials in regenerative medicine and personalized therapies. Even veterinary medicine could benefit, with applications for managing arthritis in animals.
For RA patients, this breakthrough represents a significant step toward more effective, less burdensome treatment options. By eliminating the need for frequent injections and reducing systemic toxicity, this innovative drug delivery system offers renewed hope for those battling the daily challenges of rheumatoid arthritis.