 Breaking the Old Rules
Breaking the Old Rules
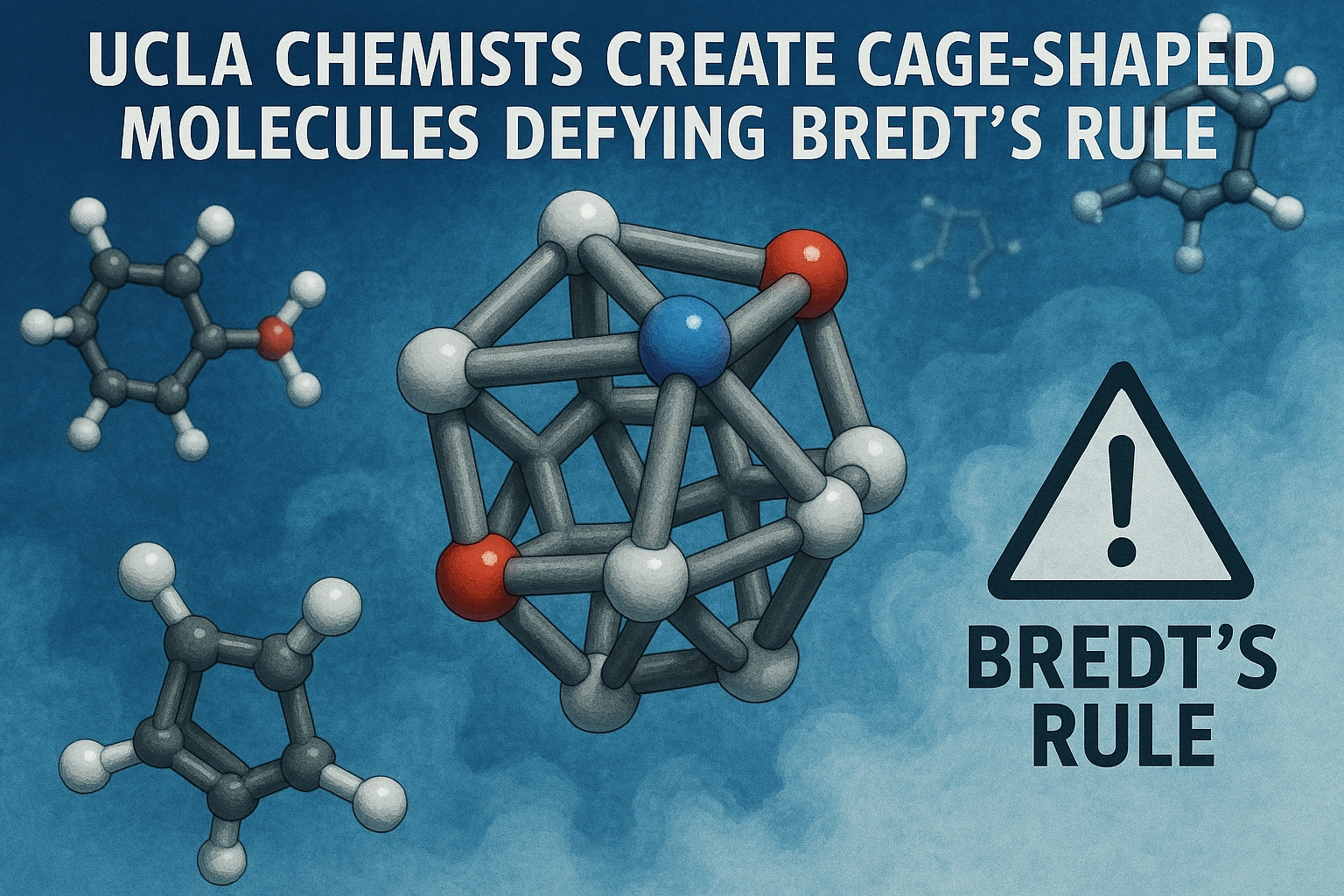
Traditionally, Bredt’s rule states that double bonds cannot exist at the bridgehead of a bicyclic molecule. Neil Garg’s lab at UCLA first challenged this in 2024 and has now extended the chemistry to more complex cage-shaped molecules. The molecules exhibit “hyperpyramidalized” geometries, with bond orders around 1.5 instead of the typical 2, making them structurally unique.
Implications for Drug Discovery
These novel molecules provide a three-dimensional scaffold for the design of future drugs. Unlike traditional flat molecules, cubene and quadricyclene offer rigidity and 3D complexity, which could enable the development of more potent and selective pharmaceuticals. Garg emphasizes that pushing the boundaries of chemical knowledge is essential for innovation in medicine.
How the Molecules Were Synthesized
The team created stable precursors containing silyl and leaving groups. Treating these with fluoride salts generated cubene and quadricyclene, which were intercepted with additional reactants to produce complex, otherwise difficult-to-make products. The distorted geometries allow these reactions to proceed rapidly, despite the inherent strain in the molecules.
Future Prospects
Although highly strained and short-lived, cubene and quadricyclene demonstrate that chemists can expand beyond conventional molecular rules. Garg and collaborator Ken Houk believe these molecules will inspire new synthetic pathways and drive the next generation of drug discovery. The research highlights the importance of questioning traditional rules to unlock novel chemical possibilities.
The study, Hyperpyramidalized alkenes with bond orders near 1.5 as synthetic building blocks, was published in Nature Chemistry and represents a major step forward in organic chemistry and pharmaceutical research.




