The Indian delegation, led by Joint Secretary Monalisha Dash, included senior officials from Central research councils and advisory bodies — among them Prof. (Dr.) Rabinarayan Acharya (CCRAS), Dr. Subhash Kaushik (CCRH), and other technical experts. On the German side, senior officials from the Ministry of Health, Charité Berlin and statutory insurance and regulatory bodies participated, reflecting a cross-sectoral approach to engagement.
Three pillars of cooperation
Deliberations at the meeting centred on three priority pillars: integrating traditional medicine into public health systems, establishing reimbursement pathways for patient access, and strengthening regulatory approval mechanisms. Both sides emphasised an evidence-based pathway that safeguards safety, quality and efficacy while improving patient choice.
Participants reviewed possible mechanisms for integrating Ayush interventions into clinical pathways, and discussed pilot projects for integrative care, particularly in community and hospital settings. The discussions sought to balance clinical rigour with cultural competence and patient preference.
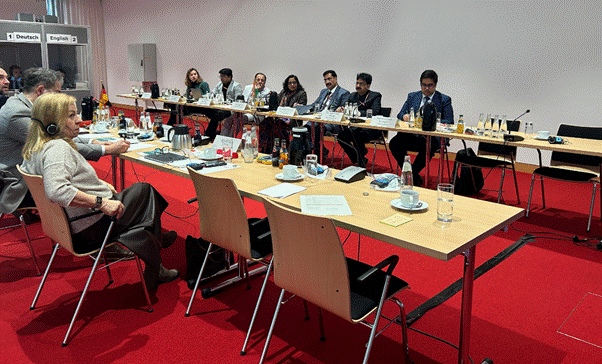 Institutional visits and engagement
Institutional visits and engagement
The mission included targeted visits to leading German institutions to explore research collaboration and practical models of integration. Key stops were the Competence Center for Traditional and Integrative Medicine at Charité University, the Community Hospital Havelhöhe (Clinic for Anthroposophic Medicine) and meetings with the Federal Joint Committee (G-BA) to study insurance and reimbursement frameworks.
These engagements aimed to map how integrative therapies can be evaluated, reimbursed and regulated within Germany’s statutory insurance framework — insights that Indian delegates said would be valuable in shaping India’s own pathways for patient access and insurance coverage.
Regulatory and research pathways
Officials discussed harmonising regulatory approaches to traditional medicine products and practice standards. The Federal Institute for Drugs and Medical Devices (BfArM) and German clinical stakeholders flagged the importance of transparent approval processes, pharmacovigilance and standardisation of clinical evidence.
India’s representatives underlined ongoing efforts to strengthen research institutions such as CCRAS and CCRH and to scale clinical trials, observational studies and collaborative research that document outcomes and safety profiles for Ayush interventions.
Reimbursement and patient access
Insurance and reimbursement formed a key strand of talks. Representatives from German statutory health insurance (including BKK mkk) engaged with the Indian team to unpack models where integrative therapies are reimbursed under defined clinical pathways. Both sides explored pilot reimbursement designs that protect patients while supporting evidence generation.
Delegates noted that establishing clear reimbursement pathways would be essential to broaden patient access and to create sustainable economic models for integrative services in public and private healthcare settings.
Strategic outcomes and next steps
The meeting concluded with a shared commitment to continue collaborative work on research, regulatory alignment and pathways for reimbursement. Both nations signalled intent to pursue joint research projects, technical exchanges and capacity-building activities that can underpin evidence-driven integration.
The Ministry of Ayush said the engagement will support India’s strategic goal to globalise validated Ayush systems while ensuring safety and scientific credibility. German partners emphasised the need for rigorous methodologies and transparent regulatory frameworks as the foundation for expanded cooperation.